
We can imagine that the fish is our closed system, any changes will not occur in it (the fish will not pull itself out of the pond) without our external influence and participation (efforts).Īn interesting fact: the first law of thermodynamics explains why all many attempts to invent the “perpetual motion” had failed. These folk proverbs can serve as a peculiar expression of the first law of thermodynamics – “no water flows under a recumbent stone”, “you can’t easily pull a fish out of a pond” and so on. It is necessary to apply certain efforts from outside in order for some changes to occur in the system. Work done by somebody else is one less thing we have to take care of.The simplest definition of the first law of thermodynamics may sound like this: a change in the internal energy of a system is possible only under external influence. Work done by the system is negative and work done on the system is positive. To calculate efficiencies, we have to use the right sign convention.


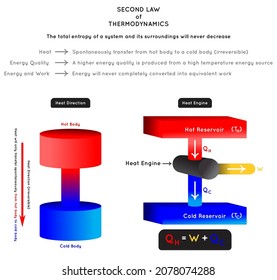
No heat engine is 100% efficient (not even close) because of the energy lost to Q C: doing work by engine requires heat (energy) to flow to the sink, Q C, from the heat source, Q H In other words, if some heat doesn't travel to Q C, there can't be any work done at all. Some of the heat energy Q H put into the system is always lost as Q C. Now we can rewrite the efficiency formula in terms of only Q H and Q C, by taking and substituting in the equation for Q H above to create, and then reducing that to. If no energy is lost from the system due to, say, friction, the energy put into the system, Q H, is turned into work, W, and the waste heat, Q C. Heat engines, like everything else in the universe, follow the First Law of Thermodynamics: energy must be conserved. The efficiency, ε, is the ratio of the work done by the engine, W, to the amount of heat energy that was put into the system from the hot reservoir, Q H. So how efficient are heat engines? This varies a bunch, but (lucky for us) the efficiency is calculated using a short formula. Recycling!īehold the deceptively peaceful exterior of a power plant. A condenser turns the wasted heat, or the steam that wasn't used to make the turbine turn, back into water, which is then pumped back into the boiler to be reused. The steam rises, blows off some, well, steam, and turns a turbine to perform mechanical work. Heat is transferred from a furnace or boiler to make steam. It's kind of like how the flow of water downstream turns the wheel of a watermill, but not nearly as picturesque.Ī steam power-plant is a kind of heat engine too. The flow of heat from the high temperature reservoir to the low temperature reservoir is used to do mechanical work. A car motor is a heat engine so is a steam engine on one of those riverboats on the Mississippi River, along with an old-school whistling tea kettle.Ī heat engine is composed of a high internal energy (high temperature) reservoir and a low internal energy reservoir. "Heat engine" is a catch-all term physicists and engineers use when they talk about a system where the transfer of heat is used to perform work. Statement (2) makes sense when we consider heat engines. Energy, or heat, flows from the high internal energy countertop to the low energy ice cream sandwich. The granite molecules have all this extra internal energy that they can share with the less energetic ice cream molecules, warming the ice cream while simultaneously cooling the granite beneath it. The ice cream sandwich is cold (The freezer is to blame). The counter is warm because it has a higher internal energy, with faster molecular motion. Statement (1) says that when a warm countertop melts your ice cream, it's because energy, or heat, moves from the kitchen counter to the ice cream. Let's look at both of these statements in a bit more detail. That first part doesn't sound too bad, but the second means we're always falling further and further behind on our work. It says that when we try to apply the First Law in the real world that (1) energy-in the form of heat-always flows from hot to cold, and (2) that even though energy is conserved, we can never turn all the heat energy into work. The Second Law comes along and reins in the First, crushing our hopes and dreams. It's all pretty zen, if you think about it. We haven't made any gains, sure, but nothings been lost, either.

No matter what happens, at the end of the day, our energy is going to be conserved. The First Law of Thermodynamics had a lesson for us-everything is even and fair. Rein it in, Second Law of Thermodynamics.


 0 kommentar(er)
0 kommentar(er)
